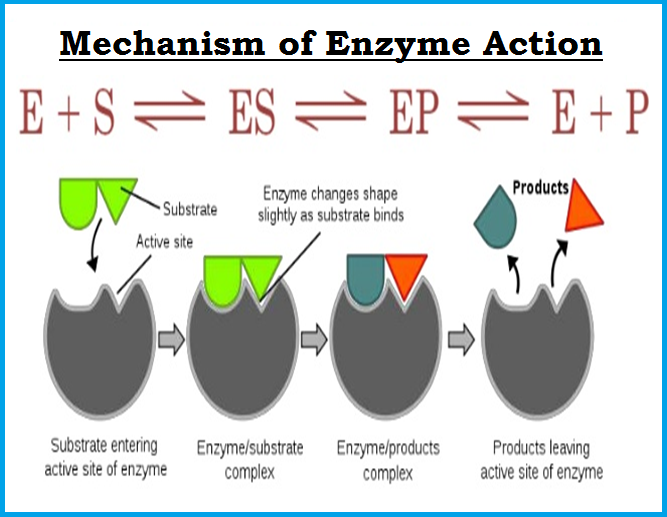
● The `color{violet}("activity of an enzyme")` can be affected by a change in the conditions which can alter the `color{violet}("tertiary structure")` of the `color{violet}("protein.")`
● These include `color{brown}("temperature, pH, change in substrate concentration")` or binding of specific `color{brown}("chemicals")` that regulate its activity.
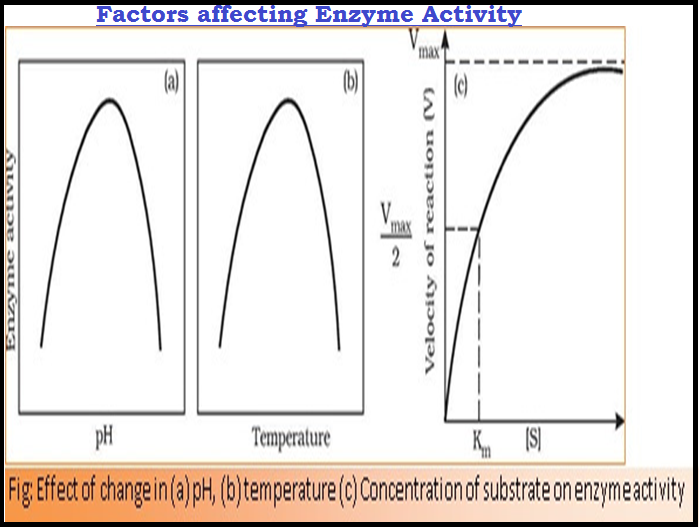
`color{green}(star "Temperature and pH")`
● `color{violet}("Enzymes")` generally function in a narrow range of `color{violet}("temperature and pH.")`
● Each enzyme shows its highest activity at a particular `color{violet}("temperature and pH")` called the `color{brown}("optimum temperature")` and `color{brown}("optimum pH.")`
● Activity declines both below and above the `color{violet}("optimum value.")`
● Low temperature preserves the `color{violet}("enzyme ")` in a temporarily `color{violet}("inactive state")` whereas high temperature `color{violet}("destroys enzymatic activity")` because proteins are `color{brown}("denatured")` by `color{violet}("heat.")`
`color{green}(star "Concentration of Substrate")`
● With the increase in substrate concentration, the `color{violet}("velocity of the enzymatic reaction")` rises at first.
● The reaction ultimately reaches a `color{brown}("maximum velocity" (V_("max")))` which is not exceeded by any further rise in concentration of the substrate.
● This is because the `color{violet}("enzyme molecules")` are fewer than the `color{violet}("substrate molecules")` and after saturation of these molecules, there are no free `color{violet}("enzyme molecules")` to bind with the additional substrate molecules.
`color{green}(star "Presence of Inhibitors")`
● The `color{violet}("activity of an enzyme")` is also `color{violet}("sensitive")` to the presence of specific chemicals that `color{violet}("bind to the enzyme")`.
● When the `color{violet}("binding of the chemical shuts off enzyme activity")`, the process is called `color{brown}("inhibition")` and the chemical is called an `color{brown}("inhibitor.")`
● When the `color{violet}("inhibitor")` closely resembles the substrate in its `color{violet}("molecular structure")` and `color{violet}("inhibits the activity of the enzyme")`, it is known as `color{brown}("competitive inhibitor.")`
● Due to its close structural similarity with the substrate, the `color{violet}("inhibitor competes")` with the substrate for the substrate `color{violet}("binding site of the enzyme.")`
● Consequently, the substrate cannot bind and as a result, the `color{violet}("enzyme action")` declines, e.g., `color{violet}(" inhibition")` of `color{brown}("succinic dehydrogenase by malonate")` which closely resembles the substrate `color{brown}("succinate")` in structure.
● Such `color{violet}("competitive inhibitors")` are often used in the control of `color{brown}("bacterial pathogens.")`
● The `color{violet}("activity of an enzyme")` can be affected by a change in the conditions which can alter the `color{violet}("tertiary structure")` of the `color{violet}("protein.")`
● These include `color{brown}("temperature, pH, change in substrate concentration")` or binding of specific `color{brown}("chemicals")` that regulate its activity.
`color{green}(star "Temperature and pH")`
● `color{violet}("Enzymes")` generally function in a narrow range of `color{violet}("temperature and pH.")`
● Each enzyme shows its highest activity at a particular `color{violet}("temperature and pH")` called the `color{brown}("optimum temperature")` and `color{brown}("optimum pH.")`
● Activity declines both below and above the `color{violet}("optimum value.")`
● Low temperature preserves the `color{violet}("enzyme ")` in a temporarily `color{violet}("inactive state")` whereas high temperature `color{violet}("destroys enzymatic activity")` because proteins are `color{brown}("denatured")` by `color{violet}("heat.")`
`color{green}(star "Concentration of Substrate")`
● With the increase in substrate concentration, the `color{violet}("velocity of the enzymatic reaction")` rises at first.
● The reaction ultimately reaches a `color{brown}("maximum velocity" (V_("max")))` which is not exceeded by any further rise in concentration of the substrate.
● This is because the `color{violet}("enzyme molecules")` are fewer than the `color{violet}("substrate molecules")` and after saturation of these molecules, there are no free `color{violet}("enzyme molecules")` to bind with the additional substrate molecules.
`color{green}(star "Presence of Inhibitors")`
● The `color{violet}("activity of an enzyme")` is also `color{violet}("sensitive")` to the presence of specific chemicals that `color{violet}("bind to the enzyme")`.
● When the `color{violet}("binding of the chemical shuts off enzyme activity")`, the process is called `color{brown}("inhibition")` and the chemical is called an `color{brown}("inhibitor.")`
● When the `color{violet}("inhibitor")` closely resembles the substrate in its `color{violet}("molecular structure")` and `color{violet}("inhibits the activity of the enzyme")`, it is known as `color{brown}("competitive inhibitor.")`
● Due to its close structural similarity with the substrate, the `color{violet}("inhibitor competes")` with the substrate for the substrate `color{violet}("binding site of the enzyme.")`
● Consequently, the substrate cannot bind and as a result, the `color{violet}("enzyme action")` declines, e.g., `color{violet}(" inhibition")` of `color{brown}("succinic dehydrogenase by malonate")` which closely resembles the substrate `color{brown}("succinate")` in structure.
● Such `color{violet}("competitive inhibitors")` are often used in the control of `color{brown}("bacterial pathogens.")`